Home
electronic Questionnaire on Quality of Life with Hidradenitis Suppurativa
eQoL-HS
Quality of life assessment with acne inversa made easy and quick
What is the eQoL-HS?
The eQoL-HS is the electronic version of the Quality of Life with Hidradenitis Suppurativa (QoL-HS). It is a patient-reported outcome measure that has been thoughtfully developed and validated by Otten et al. in 2023 to assess the impact of Hidradenitis Suppurativa (HS), also known as acne inversa, on the quality of life experienced by patients. This comprehensive tool aims to incorporate the valuable perspective of patients into treatment decisions and gain a deeper understanding of the patients’ impairments. The QoL-HS is designed to be applicable in both clinical practice and clinical trials, ensuring its practicality and relevance in various settings. By utilizing this patient-reported outcome measure, healthcare professionals can gain valuable insights into the lived experiences and impairments faced by individuals with HS, enhancing the provision of personalized care and advancing research in the field.
Validation of questionnaire
paper version: yes digital version: no
How to use the eQoL-HS
The QoL-HS is a patient-reported outcome measure that allows patients to easily complete the questionnaire at home or in a clinical setting. It consists of 23 questions designed to assess the impact of acne inversa on quality of life over the past week. Patients provide responses using a 5-point Likert scale, ranging from "not at all" to "very much" (scored from 0 to 4). To calculate the QoL-HS score, the average of all items is computed, with higher scores indicating greater impairment due to HS.
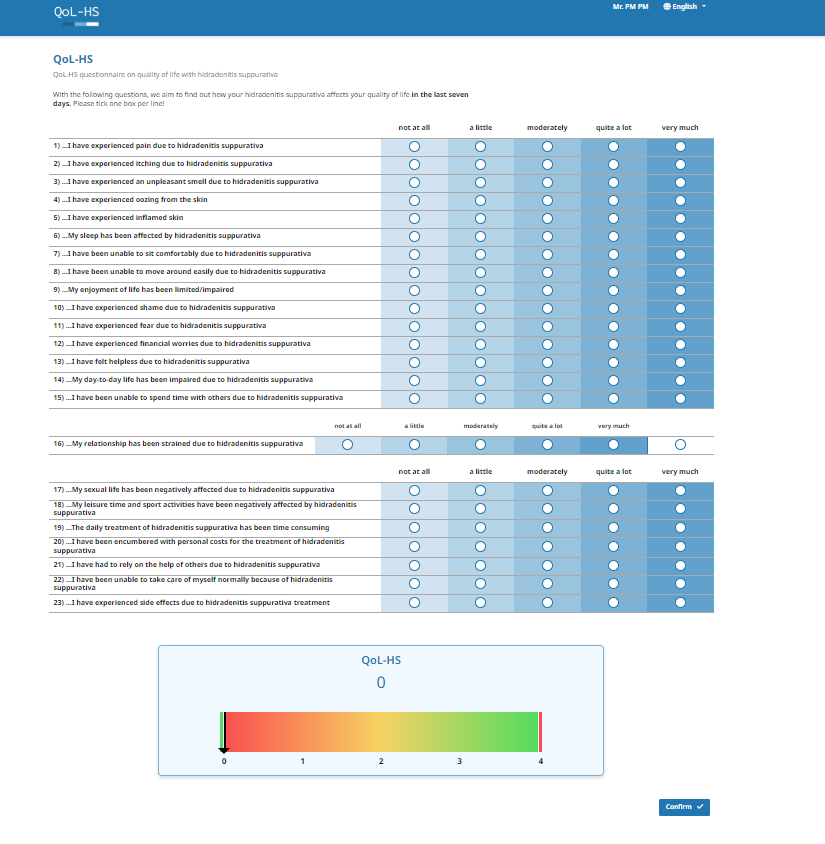
The following versions of the eQoL-HS are available
Why should quality of life be assessed in patients with hidradenitis suppurativa?
Assessing quality of life is crucial in evaluating acne inversa due to its chronic and impactful nature. It allows for patient-centered care by understanding the unique experiences and limitations faced by individuals with HS. Measuring quality of life guides treatment decisions, considering the diverse severity and impact of HS on physical, psychological, and social aspects. It serves as an important outcome measure in research, assessing treatment effectiveness beyond physical manifestations. Furthermore, quality of life assessment raises awareness about HS and advocates for improved care and support. By incorporating quality of life, healthcare professionals address the multidimensional impact of HS, personalize care, inform treatment decisions, advance research, and promote patient well-being.
Features
USER-FRIENDLY
The eQoL-HS is self-explanatory and easy to use.
QUICK
Save time with our online tool. Fill in the questionnaire and your eQoL-HS score will be calculated automatically.
INFORMED DECISION-MAKING
The eQoL-HS enables the physician to make informed treatment decisions based on patient needs.
MULTILINGUAL
The eQoL-HS is available in different languages. The use of the respective first language reduces language barriers and supports the understanding between physician and patient.
MONITOR IMPAIRMENT
Use the eQoL-HS to recognize low quality of life in time.
ACCESSIBLE FROM ALL DEVICES
With DermaValue, your eQoL-HS is always just a few clicks away. The application is available for PCs, tablets, smartphones, and other devices.
PATIENT-CENTRED
Results from the eQoL-HS provide a patient-orientated analysis of the impact that acne inversa has on patients' lives.
VALIDATED
Studies have shown that the QoL-HS is a valid indicator for quality of life with acne inversa.
ENVIRONMENTALLY FRIENDLY
Take care of the environment and save your patient data on the online platform or as PDF files for yourself and your physicians.
Reference
Otten, Marina; Augustin, Matthias; Blome, Christine; Topp, Janine; Niklaus, Marina; Hilbring, Caroline et al. (2023): Measuring Quality of Life in Hidradenitis Suppurativa: Development and Validation of a Disease-specific Patient-reported Outcome Measure for Practice and Research. In: Acta dermato-venereologica 103, adv00859. DOI: 10.2340/actadv.v102.2485 .
Our other tools
Our partners






